Absolute Temperature and Ideal Gas Equation
Absolute Temperature and Ideal Gas Equation: Overview
This topic covers concepts such as Boyle's law, Charles' law, equation of state for ideal gas, triple point, and absolute zero temperature.
Important Questions on Absolute Temperature and Ideal Gas Equation
of a gas at a pressure of is compressed to . Taking the temperature to remain constant, the increase in pressure, is
Under what conditions will a pure sample of an ideal gas not only exhibit a pressure of but also a concentration of ?
Select one correct statement. In the gas equation, PV = nRT
The temperature shift of hydrogen gas in a container is to . The gas expanded without any external heat gain or lose, the initial pressure was , what will be the pressure at that gas in ?
An air bubble of volume rises from the bottom of a lake deep to the surface at a temperature of The atmospheric pressure is the density of water is and There is no difference of the temperature of water at the depth of and on the surface. The volume of air bubble when it reaches the surface will be
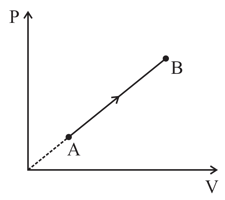
In the given diagram, where andare pressure and volume of an ideal gas respectively. Then
Temperature of moles of an ideal gas is increased from to through a process ( is constant). Find the work done by gas.
An air bubble having volume at depth inside water comes to surface. What will be the volume of the bubble at the surface?
In rising from the bottom of a lake, to the top, the temperature of an air bubble remains unchanged, but its diameter gets doubled. If is the barometric height (expressed in of mercury of relative density ) at the surface of the lake, the depth of the lake is
At the density of a gaseous oxide atbar is the same as that of nitrogen (Atomic mass =) at bar. Calculate the molar mass of the oxide
What is the work done by mole of a gas at room temperature to double its volume during isobaric process? (Take, )
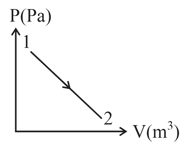
The figure shows diagram for a given mass of an ideal gas for the process . During this process temperature of the gas
Given,on increasing the value of by , the value of increases by, then find initial value of :-
When an air bubble of radiusrises from the bottom to the surface of a lake, its radius becomes . Taking the atmospheric pressure to be equal to height of water column, the depth of the lake would approximately be (ignore the surface tension and the effect of temperature)
One mole of an ideal monoatomic gas is taken at a temperature of . Its volume is doubled keeping its pressure constant. Find the change in internal energy.
What is Boyle law explain in detail?
Two flasks and of volume and contain same gas at pressure and respectively at the same temperature. Pressure of the gas when the flasks and are connected by a tube of negligible volume is
Statement (I) : Gas thermometers are less sensitive than liquid thermometers.
Statement (II) : The ratio of universal gas constant and avagadro's number is called Boltzman's constant.
Statement (III) : The density of a given mass of a gas at constant pressure is inversely proportional to its absolute temperature.
The correct option among the following is
Some nitrogen is held in tank at a pressure of . The tank is connected to a tank that is completely empty (evacuated), and valve is opened to connect the two. Determine the total pressure in the two-tank system after the nitrogen stops flowing. Assume that no temperature change occurs during the process.
An empty pressure cooker of volume liters contains air at atmospheric pressure a and temperature of . It contains a whistle which has area of and weight of . What should be the temperature of air inside so that the whistle is just lifted up?